Chapter 5: Laboratory Biosafety Practices
Basic Laboratory Practices
The following prudent biosafety practices are recommended by the National Academy of Sciences in the publication Biosafety in the Laboratory and in part constitute basic good biosafety practices. Although these practices may be considered “common sense” and overly simplistic by experienced laboratorians, strict adherence to these basic principles will greatly reduce the likelihood of laboratory acquired infections.
| Biosafety Practice | Routes of Exposure Blocked |
|---|---|
| Do not mouth pipette | Inhalation, ingestion, skin and mucous membrane contact |
| Manipulate infectious fluids carefully to avoid spills and the production of aerosols | Inhalation, skin and mucous membrane contact |
| Restrict use of needles, syringes, and other sharps to those procedures for which there are no alternatives; dispose of sharps in leak- and puncture-proof containers | Percutaneous, inhalation |
| Use lab coats, gloves, safety eye wear, and other personal protective equipment | Inhalation, skin and mucous membrane contact |
| Wash hands after all laboratory activities, following the removal of gloves, and immediately following contact with infectious agents | Ingestion, skin and mucous membrane contact |
| Decontaminate work surfaces before and after use, and immediately after spills | Ingestion, skin and mucous membrane contact |
| Do not eat, drink, store foods, or smoke in the laboratory | Ingestion, skin and mucous membrane contact |
Biological Hazard Information
Laboratory workers must be knowledgeable of the hazards associated with the biological agents present in the laboratory, and have hazard information available to them. The following are sources of hazard information for biological agents.
Microbial Agents
- The CDC/NIH publication Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition has descriptions of biosafety levels and recommended biosafety practices for specific biological agents.
- The Public Health Agency of Canada publishes Pathogen Safety Data Sheets that describe hazardous properties of human pathogens and recommendations for laboratory work.
- The American Biological Safety Association (ABSA) maintains a database for Risk Group Classification for Infectious Agents.
Toxins
Purified biological toxins are chemical hazards, although many such toxins produce adverse effects at doses significantly below that of “traditional” laboratory chemicals. Laboratory use of purified toxins falls under the University of Nevada, Reno Chemical Hygiene Plan, and Safety Data Sheets (SDSs) must be maintained and available.
- SDSs for the specific toxin should be received from the vendor upon receipt of the toxin.
- Toxicology textbooks such as Casarett’s and Doull’s Toxicology are also good sources of hazard information for some toxins.
- The BMBL 6th Edition also contains information on biological toxins and associated safe work practices.
Written Standard Operating Procedures
This manual, in combination with the referenced CDC/NIH publications Biosafety in Microbiological and Biomedical Laboratories and Guidelines for Research Involving Recombinant and Synthetic Nucleic Acid Molecules, provides general standard operating procedures (SOPs) for working with biological agents. However, because these SOPs cover relatively general topics, individual laboratories are required to develop laboratory specific SOPs that cover the biosafety concerns and laboratory procedures for that particular laboratory. For example, laboratory-specific SOPs should address safe manipulation of specific organisms, specific exposure control methods, and specific decontamination and waste handling requirements. There is no standard format required for SOPs and laboratories are encouraged to use any format that effectively conveys the biosafety information (including use of pictures and illustrations). The laboratory-specific SOPs do not need to duplicate the more general SOPs contained in this manual but should supplement this document.
Security and Inventory of Biological Agents
Each PI is responsible for ensuring that his or her laboratory implements sufficient security measures and procedures to prevent unauthorized access to biological agents. Select Agents (see Chapter 14) and other higher risk microorganisms and toxins must be stored in a locked container, and an inventory must be maintained with sufficient detail to enable identification of missing materials.
Prevention and Containment of Aerosols and Droplets
Handling of liquids or dry powders is likely to generate aerosols or droplets. Procedures such as centrifuging, mixing, and pipetting that involve high energy tend to produce respirable aerosols that stay airborne for extended periods and are small enough to be inhaled. Low energy procedures including opening containers and streaking plates produce droplets that settle quickly on surfaces, skin, and mucous membranes.
Biological Safety Cabinets
Procedures involving infectious material should be performed inside a biological safety cabinet (BSC) whenever possible. A properly operating, properly used BSC (see Chapter 8) will contain aerosols and droplets generated during handling of infectious agents.
Pipetting
Do not mouth pipette! Always use a mechanical pipetting device. Pipettes should be drained gently with the tip against the inner wall of the receiving vessel and liquid should not be forcibly expelled from the pipette.
Blending
Use a safety blender that has leak proof bearings and a tight-- fitting lid with a sealable gasket. Use the blender inside a BSC when blending material containing infectious agents.
Centrifugation
The potential for contamination and infection is high if liquid and aerosol is released during centrifugation. Sealed centrifuge buckets, or safety cups should be used to prevent release of liquid and aerosol. Ultracentrifuges operate under vacuum and should contain an in-line HEPA filter between the chamber and the vacuum pump. Small bench top centrifuges can be placed inside a BSC to contain infectious aerosols and prevent personnel exposures.
When centrifuging BSL-3 agents outside of a BSC, approved respiratory protection should be worn when opening the centrifuge as a precaution against possible release of the agent during centrifugation. Rotors containing infectious agents must be loaded and unloaded (or otherwise opened) in a BSC.
Inoculating Loops
Flaming inoculating loops can result in spatter and release of aerosols and droplets. Use of an electric microincinerator will effectively control spatter resulting from sterilization of inoculating loops.
Use of Absorbent Materials
Work surfaces should be covered with absorbent paper or “diaper” sheets to collect splashes and drips, and minimize the spread of contamination. The absorbent paper should be changed at the end of the laboratory procedure as part of the final cleanup, or at least daily during use.
Personal Protective Equipment
Although not a substitute for use of BSCs and good laboratory practices, personal protective equipment (PPE) is considered a primary barrier to infectious agents and proper use will reduce the likelihood of infection. PPE is the least desirable exposure control method because its failure results in direct exposure to the agent. PPE is most effective when used to supplement primary control methods such as biological safety cabinets, safety centrifuge cups, and other containment devices. For example, manipulation of infectious agents that have a very low infectious dose and which are readily transmitted by inhalation of aerosols, such as Mycobacterium tuberculosis, may necessitate the use of respiratory protection in addition to containment within a BSC.
Laboratory Coats and Gowns
Laboratory coats protect street clothes against chemical and biological spills, and provide additional body protection. Generally, a 100% cotton lab coat is recommended over polyester-cotton blends for general microbiological work. The wearing of lab coats is considered to be standard microbiological practice for BSL-1 and BSL-2 laboratories. For BSL-3 laboratories, CDC/NIH guidelines recommend solid-front or wrap-around gowns or suits, rather than front-buttoning lab coats. It is good laboratory practice (and a requirement for BSL-3 labs) to remove lab coats or gowns before leaving the laboratory to minimize the spread of contamination outside the laboratory. Lab coats should be left in the laboratory and must not be taken home for washing.
Gloves
Gloves are available that provide protection against a variety of hazards, including infectious agents, chemicals, and radioactive material. Unfortunately, there is no single glove type that provides adequate protection for all hazards (or even all chemicals!). Always check gloves for pinholes prior to use.
Standard latex examination type gloves provide protection against microbiological hazards, including human blood and body fluids. Latex gloves do not generally provide adequate protection against liquid chemicals; additionally, many people develop latex allergies as a result of wearing latex gloves. Disposable nitrile gloves are an alternative to latex examination gloves that provide similar dexterity and increased chemical resistance. Nitrile gloves still provide protection against microbiological hazards, but without the latex allergy hazard. Although disposable nitrile gloves generally provide better chemical protection than latex gloves (for example, nitrile gloves are recommended when handling tamoxifen), they are not considered to be chemical resistant gloves. For instances where chemical contact is likely or cannot be tolerated due to high toxicity, consult the University Chemical Hygiene Plan or contact the University Chemical Hygiene Officer (Luis Barthel-Rosa) at (775) 327-2270 or the University Biosafety Officer (Keith Kikawa) at (775) 327-5046 for recommendations concerning chemical resistant gloves.
Glove selection for protection against radioactive materials should be based on the resistance of the glove to the liquid solvent that the radioactive material is contained in. Essentially any rubber-type glove material will provide protection against dry chemicals or radioactive material. For more information about radiation safety and control of radioactive contamination, contact the University Radiation Safety Officer (Myung Chul Jo) at (775) 784-4540.
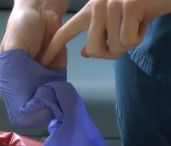
Contamination control requires that gloves be removed prior to exiting a BSC or touching non-contaminated laboratory areas and equipment (such as clean areas, phones, computers, door knobs, etc.). Be careful to remove gloves from the inside out so as to minimize contact between outside of gloves and bare skin during removal (to prevent self-contamination). The proper procedure for removing gloves is shown in photos below. If you have any issues accessing this content, please contact EH&S.
Proper removal of potentially contaminated gloves:
- Using one gloved hand, pinch and pull the base of the other gloved hand.
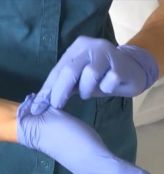
- Use the middle finger to scoop the cuff off the glove.
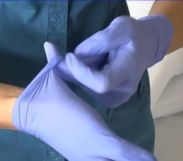
- Pull the glove inside out over all the fingers and thumb to form a "beak."
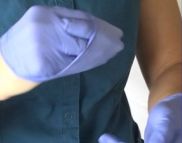
- With the beaked hand, pinch the opposite glove at the base and pull the cuff.
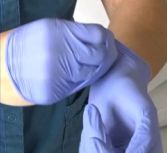
- Roll the glove inside out and off the hand.

- With the gloved hand, use the index finger to pull the beaked glove off at the base of the beak and dispose into the appropriate waste container. Always wash your hands after glove removal.
Eye and Face Protection
Safety glasses, goggles, and face shields provide protection against chemical reagents and disinfectants. Additionally, they also provide protection against infection that can result from the splashing of pathogenic organisms into the eyes. Normal prescription eyeglasses are not safety glasses and do not provide adequate eye protection for laboratory operations. Further guidance on the use of protective eye and face wear for chemical hazards can be found in the University Chemical Hygiene Plan.
Goggles with indirect venting provide a good barrier against splashes to the eyes. A face shield can be worn in addition to goggles (face shields may not provide adequate eye protection by themselves) to provide protection against splashes to the face and mouth.
Respiratory Protection
Required Use of Respiratory Protection
Prior approval from EH&S is required whenever personnel are required to wear any respirator (including N-95 filtering facepiece respirators, which are also known as dust masks) to prevent exposure to infectious agents, or when required by regulation or administrative procedure. When use of respiratory protection is required, personnel must be medically evaluated, fit-tested, and trained prior to using respiratory protection. An exception to the fit test requirement is use of a powered air-purifying respirator (PAPR) with loose fitting hood; however, use of these respirators still requires a medical evaluation and training prior to initial use. The medical evaluation can be accessed completed by navigating the University Occupational Health Program page.
Voluntary Use of Respirators
Some personnel may want to voluntarily wear a N95 filtering facepiece respirator or other respirator in the absence of a recognized inhalation risk, regulatory requirement, or administrative requirement. In these circumstances, personnel are permitted to voluntarily wear a respirator; however, certain requirements must still be met so EH&S must be contacted prior to voluntary use of respirators.
Voluntary use of N-95 filtering facepiece respirators requires only that specific information contained in the applicable OSHA standard (29 CFR 1910.134, Appendix D) be provided to each person (in place or more detailed training); a medical evaluation and fit-test are not required. Filtering facepiece respirators are particulate filters and therefore are only effective against particles; they do not provide protection from inhalation of gases or vapors. Voluntary use of respirators other than a N95 filtering facepiece respirator requires a medical evaluation and training.
Respirator Fit-Tests
Contact Kacey Nidever (775-327-5192) or Crista Hartman (775-327-5055) in EH&S to schedule a respirator fit test or if there are questions about the respiratory protection program.
Storage and Labeling of Biological Agents
BSL-3 infectious agents must be stored using double containment and it is recommended that BSL-2 agents also be stored using double containment. Both the primary and secondary containers must be durable and leak proof so as to prevent accidental exposure. Primary containers must be clearly labeled to indicate the identity of the agent and include the universal biohazard symbol (see below) as physical space on the container permits. At a minimum, secondary (or outside) containers must include the universal biohazard symbol (identity of contents is also desirable). Freezers, refrigerators, and other storage areas must also be labeled with the biohazard symbol; exceptions to this policy will be considered on an individual basis by the IBC. Waste, and contaminated equipment or other objects to be decontaminated must also be labeled with the biohazard symbol.

The OSHA Bloodborne Pathogen Standard specifically requires that containers of human blood or other potentially infectious material (OPIM), contaminated waste, and refrigerators, freezers, and other storage containers used to store or transport blood or OPIM, be labeled with the universal biohazard symbol (fluorescent orange or orange-red). See the University Exposure Control Plan for additional information on handling and labeling of blood and OPIM.
Biohazard Labels and Signs
Signs must be posted at entrances to laboratories and other rooms where human, animal, or plant pathogens, or biological toxins that are listed as select agents or that have a LD50 for a mammalian species below 100 ng/kg of bodyweight, are present. A biohazard sign template is available from EH&S that can be used to communicate room-specific information.
EH&S posts signs at laboratory entrances that include the universal biohazard symbol, the biosafety level of the agents present in the room, and the name and contact information for the primary and secondary emergency contacts. Laboratory occupants should notify EH&S when any information on the door card needs to be updated. If there are any specific entry requirements (such as personal protective equipment or immunization) the laboratory occupants should post that information in a professional looking manner, or contact EH&S for assistance.
Additionally, individual cages used to house animals infected with BSL-2 or BSL-3 agents, or dosed with biological toxins listed as select agents or that have a LD50 for a mammalian species below 100 ng/kg of bodyweight, should be labeled with the biohazard symbol, biosafety level, and biological agent or toxin.