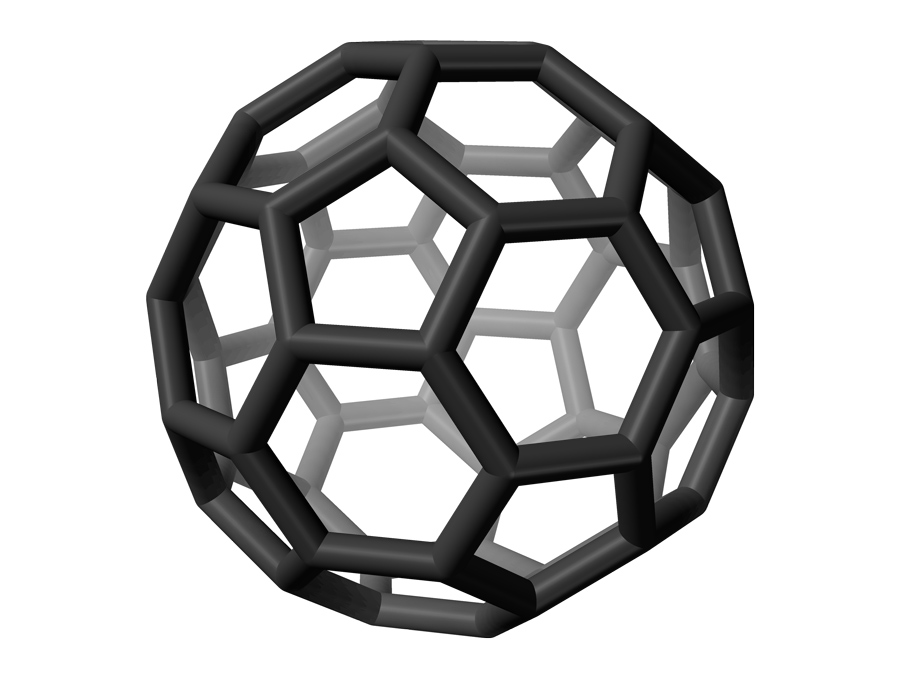
Could buckyballs be holding new quantum mysteries, which unlock their potential for quantum information science? That’s what theoretical physicist Timur Tscherbul from the University of Nevada, Reno and his collaborators are proposing in a research article published in Science. Buckminsterfullerene, known by its nickname “buckyball,” is a (relatively) large molecule, making it an unusual candidate for quantum science. This is because large molecules often display behavior which leads to loss of quantum information.
Buckminsterfullerene is composed of 60 carbon atoms organized into a truncated icosahedron. The classic soccer ball shape matches the shape of the buckyball perfectly, with each vertex on the black and white shapes representing an atom of carbon, though the soccer ball design was built before buckminsterfullerene was discovered. The shape of the molecule is interesting to chemists, physicists and mathematicians. Its shape also gives Buckminsterfullerene some rigidity, which is useful.
“This molecule is famous not only because it's so beautiful, but because it has some interesting properties,” Tscherbul said.
The troublesome behavior exhibited by molecules is known as ergodicity, which means that over time, the molecule will completely explore the space it occupies. Tscherbul gives milk and coffee as an example. When you add milk to a hot cup of coffee, the milk eventually spreads out evenly throughout the coffee. Ergodic behavior is believed to be the natural state for most large molecules. The more a molecule explores its space, the more ergodic it is, and the less promising as a container of quantum information.
Quantum computers rely on extremely stable molecules to act as quantum bits, or qubits. There has been extensive research into the possible molecules that could be used as qubits. The challenge is quantum is finicky. When environmental conditions aren’t just right, the qubits interacting with the noisy environment are susceptible to a process known as decoherence, which causes quantum information to be lost. Overcoming this obstacle and extending the lifespan of fragile quantum systems is a highly sought-after goal for dozens of experimental and theoretical groups working in the field of quantum information science.
Typically, molecules considered for quantum applications aren’t as big as Buckminsterfullerene. “The overwhelming majority of molecules that are explored for quantum applications are much smaller,” Tscherbul said. Could Buckminsterfullerene be a good candidate?
When researchers hit the buckyball with the right amount of rotational and vibrational energy, the molecule would exhibit nonergodic behavior. At certain rotational energies, Buckminsterfullerene will go back and forth between ergodic and nonergodic behavior. At a slightly different energy, however, the molecule becomes ergodic again. Tscherbul helped to calculate the energy levels of the buckyballs, which were used to model ergodic and nonergodic behaviors.
Researchers will continue to explore buckyballs as tools in the quantum toolbelt. With the article’s publication, researchers can now use the methodology to explore other molecules as potential candidates for quantum applications.
At the University of Nevada, Reno, professors across multiple departments are working on expanding the quantum toolbelt. In the Department of Physics, the experimental work conducted by Jonathan Weinstein and his group has identified atoms trapped in inert cryogenic matrices as promising quantum sensors with long coherence times. Andrei Derevianko has proposed ways to suppress decoherence in ultraprecise atomic clocks by using the so-called magic states, which are immune to decoherence, and mitigating their interactions with blackbody radiation. In the University’s Department of Chemistry, David Leitner studies non-ergodic behavior in large molecules, which leads to deviations from widely used statistical theories of chemical reaction rates. The interdisciplinary team of Sergey Varganov, Yafis Barlas, Ana de Bettencourt-Dias, Matthew Tucker, and David Cantu are exploring the potential of single-molecule magnets based on lanthanide and transition metal atoms for applications in quantum information science. Natia Frank develops optically gated molecular qubits based on transition metal ions for quantum sensing applications.