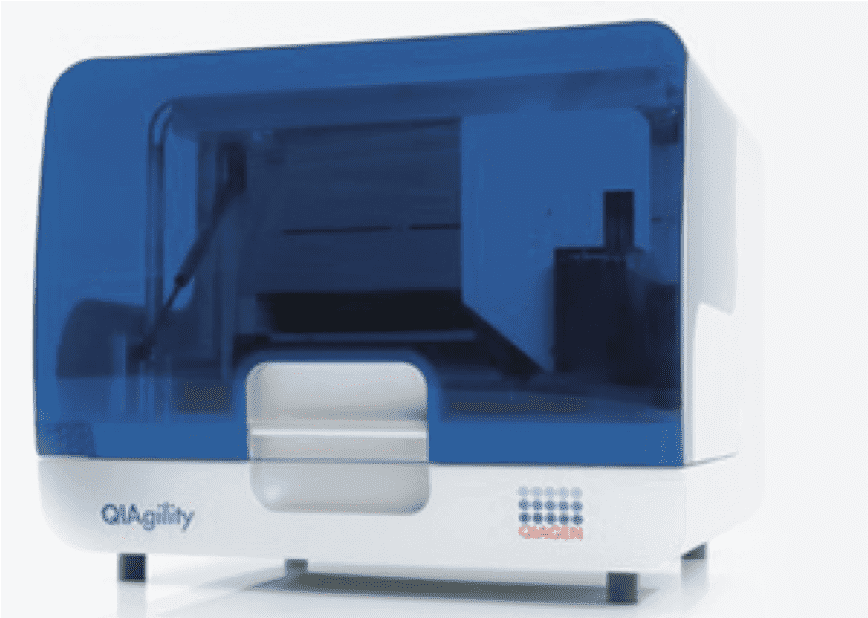
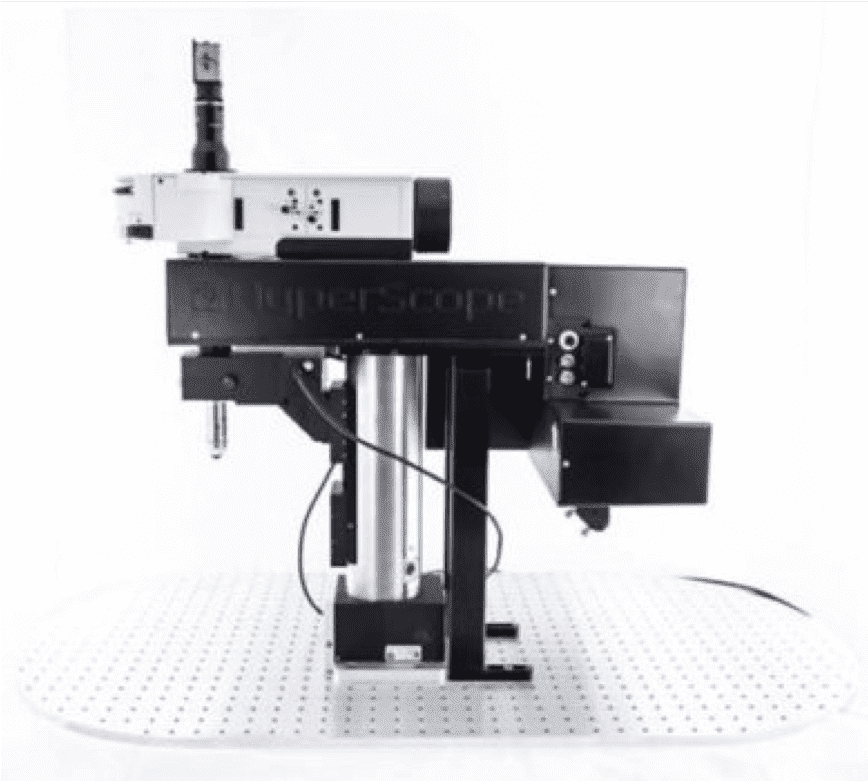
The Cellular and Molecular Imaging (CMI) core facility of the Integrative Neuroscience COBRE center provides users with instruments and services for analyzing and quantification of biomolecules, and microscopic investigation of cells, tissues, organs, and small animals with imaging capabilities for brightfield and fluorescent microscopy. The core maintains several state-of-the-art microscopy instruments including a Leica SP8 confocal with LIGHTNING capabilities, a Scientifica two-photon microscope, a Leica THUNDER 3D-tissue and a Model organism with computational-clearing capabilities. The core also maintains multiple instruments for imaging, analyzing and quantifying DNA, RNA and protein such as a Typhoon Phosphoimager, automated PCR-pipetting robot and Real-Time PCR machines. Finally, the core has modern work space and storage for the culture of insect and mammalian cells with instruments including a Microfluidic Cell Sorter and microscope systems.
We like to keep track of our scientific contributions; please send us an email when your research is published. For grant proposals, we can provide support letters and core instrument information used for your research project. To acknowledge our core funding in your publications, please use P30 GM145646 and/or P20 GM103650. Use P20 for grant years from before July 2022 and P30 after; funding that spans both terms should use both numbers.
Core Location
- Department of Biology
- Fleischmann Agriculture/Life Sciences (FA)
 Location: FA 312 (door code access required)
Location: FA 312 (door code access required)

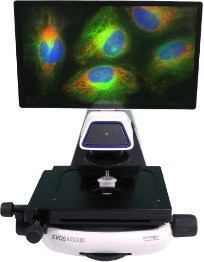



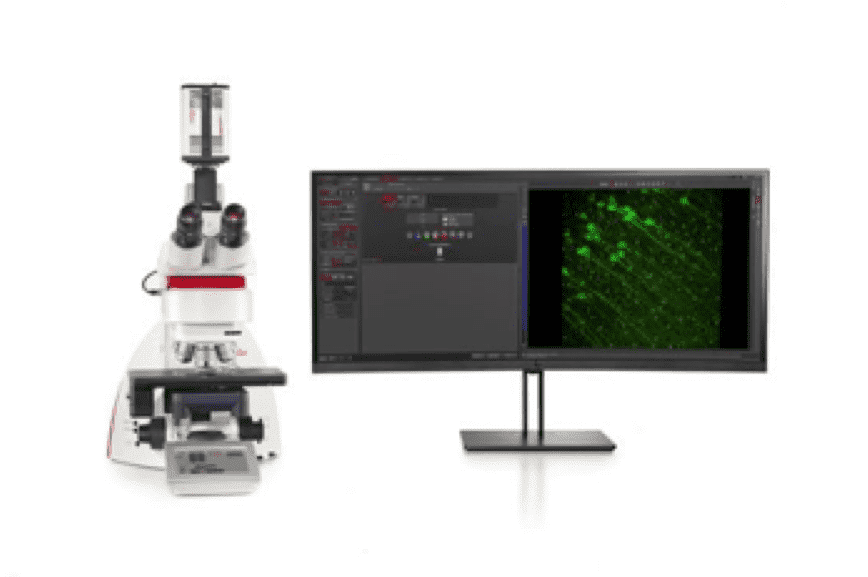 Key features of the THUNDER 3D Tissue imager include:
Key features of the THUNDER 3D Tissue imager include: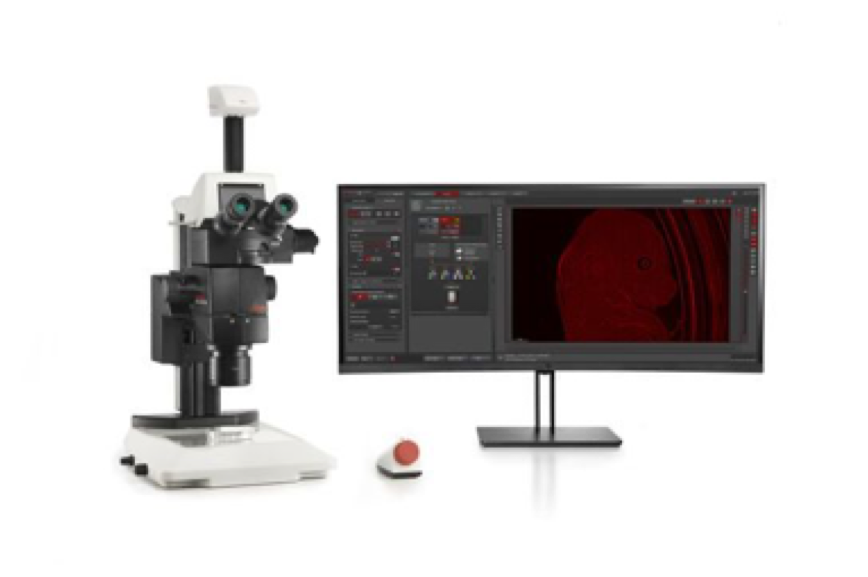 Key features of the THUNDER Model Organism imager include:
Key features of the THUNDER Model Organism imager include:
 Location: FA 314 (door code access required)
Location: FA 314 (door code access required)