Chapter 16: Packaging and Shipping Infectious Agents
Introduction
The International Civil Aviation Organization (ICAO) is a specialized agency within the United Nations that develops and maintains principles that ensure the safety of international civil aviation. The ICAO Technical Instructions for the Safe Transport of Dangerous Goods by Air are the regulations that govern the shipping of dangerous goods. These technical instructions have been incorporated into US law and are an acceptable method of transport in the US (49 CFR 171.11).
Packaging and shipping biological materials involve certain risks with numerous potential liabilities. The International Air Transport Association (IATA), normal'>Dangerous Goods Regulations (DGR), latest edition, is the worldwide gold standard for shipping. The IATA regulations apply to all air transport, both domestic and international. By following the IATA DGR you ensure that your package will also meet U.S Department of Transportation (DOT) requirements for ground transport. All responsibility for packaging and shipment of these agents have been assigned to the shipper. These include classification of shipment, proper packaging of shipment, proper marking and labeling of package, and proper documentation of shipment.
Training Requirements
Those involved in the packaging and shipping of Dangerous Goods must undergo training every two (2) years or when activities change. It is the responsibility of the department to ensure that training is completed; however, the Environmental Health and Safety Department (EH&S) can provide this training. The shipper is under obligation to receive further qualification when shipping hazardous materials of a class or division where current training is insufficient.
Classification of Shipment Type
A shipment of biological material will fall into one of the five following categories:
- Unregulated biological material
- Category A Infectious Substances
- Category B Infectious Substance
- Patient specimens
- Genetically Modified Organisms (GMOs)
Shipment Classifications
Unregulated Biological Materials
Unregulated biological materials are not subject to IATA or DOT infectious substance shipping regulations; however, these materials may require a permit for shipment abroad. Please check with EH&S if you have any question as to whether your shipment is unregulated. Some examples of unregulated biological materials include:
- Substances which do not contain infectious agents and which should not cause disease in humans or animals. These include non-infectious cells or tissue cultures; blood, plasma, or sera from humans or animals not suspected of having an infectious disease; DNA, RNA, or any other genetic elements that are not themselves infectious.
- Patient specimens that are not thought to be infectious
- Microorganisms that are not pathogenic to humans, animals, or plants.
- Substances that have been neutralized or inactivated such that they are no longer infectious.
- Environmental samples that are not known, or thought to be, infectious.
- A biological product such as an antibody or drug.
Category A Infectious Substances
Category A infectious substances are capable of causing disease in humans or animals. The proper shipping name for Category A Infectious Substances is Infectious Substance Affecting Humans (UN2814), or Infectious Substance Affecting Animals (UN2900). These substances are classified as DOT hazard class 6.2 (infectious). Links to these Category A infectious substances are found below:
Category B Infectious Substances
Category B infectious substances are materials that are infectious, but do not meet the definition of Category A infectious substances. These include patient samples, tissue cultures, and cells that are presumed to contain, or have a reasonable probability of containing, a pathogenic organism (e.g., blood known to contain HIV). The proper shipping name for these substances is "Biological Substance, Category B (UN3373)".
Genetically Modified Organisms (GMOs)
Genetically modified organisms (GMOs) are organisms in which their genetic material has been altered through recombinant DNA techniques. If a GMO is non-infectious it is classified as a DOT class 9 (miscellaneous) hazard and is assigned to UN3245. If a GMO is potentially infectious, it must be assigned as a Category A infectious agent (UN2814 or UN2900), or Category B infectious agent (UN3373).
Packaging Biological Materials
Specimen containers are to be placed in the cushioning material, which is then placed into the secondary leak-proof container. Absorbent material, sufficient to absorb the quantity of the shipment, must be placed next to the primary container. The specimens and an inventory of the contents are then placed into the outer container.
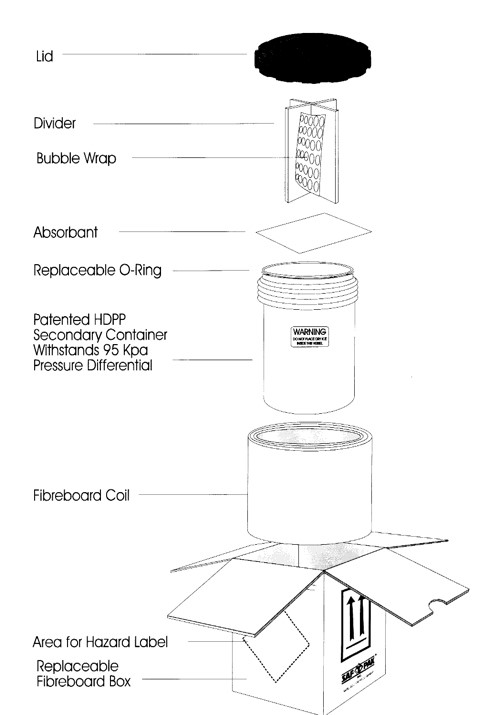
The diagram below depicts the following parts to a specimen container, in order from top to bottom:
- Lid
- Divider
- Bubble Wrap
- Absorbent Material
- Replaceable O-Ring
- Patented HDPP Secondary Container (Withstands 95 Kpa Pressure Differential)
- Fibreboard Coil
- Area for Hazard Label
- Replaceable Fibreboard Box
Containers
For Category A Infectious Substances IATA Packaging Instructions 602 apply. Three containment levels are used to package Infectious Agents for shipment. The three levels ensure containment of the agent and include:
- Primary – plastic, metal or glass leak-proof container, containing the product.
- Secondary - plastic, metal or glass leak-proof container, containing the primary container and sufficient absorbent material for the entire quantity of the product.
- Tertiary – contains secondary container, description of contents, dry ice or other preserving material, and any packaging material required to prevent shifting of contents.
Preservatives
When preservatives are shipped with a specimen or agent, the preservative must also be declared on the Shipper’s Declaration (see page 15-8). The hazard class label that applies to the preservative, and the quantity of the preservative, must also be on the outer package. Additional information may be found in the IATA Dangerous Goods Regulations, latest edition.
| Preservative | Hazard Class | UN Code | Packing Instruction |
|---|---|---|---|
| Dry Ice* | 9 | 1845 | 904 |
| Formalin, Formaldehyde Solution | 8 | 2209 | 820 |
| Nitrogen, Refrigerated Liquid | 2.2 | 1977 | 202 |
*The container must be designed to allow the release of carbon dioxide gas.
Labeling and Marking
The outer container, usually a fiberboard box, must be marked with the following information:
- Hazard Label, Class 6.2 (for all category A infectious agents)
- UN Number (see table below)
- Proper shipping name of agent, with technical name in brackets, and quantity.
- Name, address and phone number of shipper
- Name, address and phone number of receiver
- 24-hour contact phone number of responsible party (shipper)
- Two pairs of orientation arrows
Note: Labels may be obtained from the supplier of the packaging material or from other label manufacturers.
Preservative:
- Class Label (determined by the preservative used, such as Class 9 for dry ice)
- Quantity of preserving agent
- UN Shipping Code of the preservative
Example: Identification on tertiary container
5g of culture containing hepatitis B virus are to be shipped in 3 kg dry ice. The tertiary container would be labeled with the following information:
- Infectious Substance Affecting Humans (Hepatitis B virus), 5g, UN2814
- Dry Ice UN1845 3 kg
- Hazard Class Label 6, “Infectious Substance”, and Hazard Class Label 9, “Miscellaneous”, would be affixed to the container.
Note: The above information must also be located inside the tertiary (or outer) container.
Category B infectious substances are shipped utilizing IATA packaging instructions 650. The manufacturer will identify compliance with package specifications on the outer shipping container. Orientation labels may be used.
Specimen containers are to be placed in the cushioning material, which is then placed into the secondary leak-proof container. Absorbent material, sufficient to absorb the quantity of the shipment, must be placed next to the primary container. The specimens and an inventory of the contents are then placed into the outer container. The package must then be labeled with the UN3373 Label.
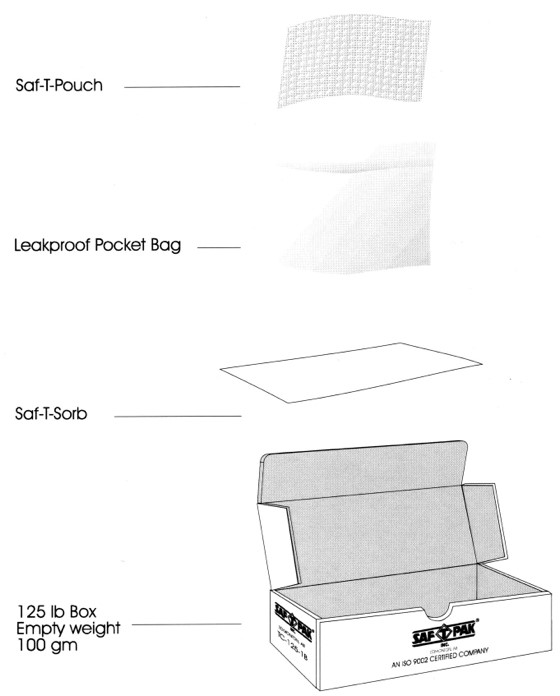
The diagram below depicts the following parts to a specimen container, in order from top to bottom:
- Saf-T Pouch
- Leakproof Bag
- Saf-T Sorb
- 125 lb Box (empty weight: 100g)
The full and accurate completion of the Shipper’s Declaration is essential to the safe transportation of the infectious agent or diagnostic specimens. These are legal documents signed by the shipper, which creates a contract between the shipper and the carrier. The document must be accurate, legible, and neat. There must be no spelling errors. The carrier will provide you with a Shippers’ Declaration and detailed instructions (see example on page 15-8). This document must be completed electronically.
- The declaration form must be completed in English.
- Three copies of the declaration must be completed. One copy will remain with the shipper (investigator). Two copies will be sent with the shipment. The following instructions refer to the Shipper’s Declaration on page 15-8.
Instructions
(Numbers correspond to the space the information should be included within.)
- Shipper’s:
- Name,
- Address,
- Phone number.
- Receiver’s:
- Name,
- Address,
- Phone number.
- Line out the item that does not apply. Passenger aircraft can only be used to ship quantities less than 50 ml. Cargo aircraft must be used to ship quantities between 50 ml and 4 L.
- Line out the item that does not apply. Radioactive or Non-Radioactive
- Identify shipment as Category A Infectious Substance.
- Identify infectious to human, animals or both
- Identify the specimen by name in parenthesis.
- Identify preserving agent*
- Class or Division *
- UN Code *
- Packaging Group * There is no packaging group for biological agents. Dry Ice is Packing Group III
- Identify the quantity
- Identify type of outer container for the shipment
- Packaging Instructions * Dry Ice is PI 904
- Must contain the following information:
- 24 hour emergency contact number for the shipper (Primary Investigator, Lab Supervisor)
- Name, Place, Date, and Signature of the shipper.
* As described in the latest edition of the IATA Dangerous Goods Regulations